* indicates required field
VTR Request Process and Documents
Investigators interested in using the SEER-Linked VTR Program to acquire de-identified, linked tissue, clinical data, pathology reports, and/or digital whole slide images (WSIs) are required to complete the following submission and review process (Figure 1).
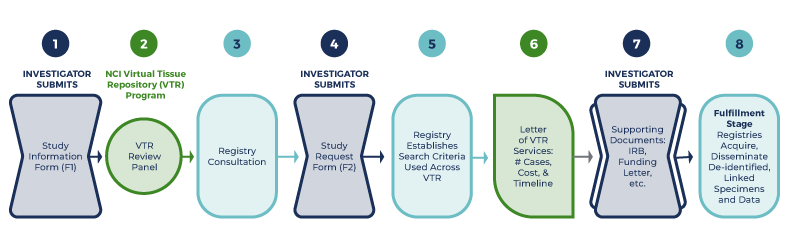
- Study Information Form (F1): The investigator creatres a log in to the VTR Request System and completes and submits the Study Information Form (F1) in the VTR Request System.
- VTR Program Review Panel: meets to decide if study is approved, conditionally approved pending further information, or declined. If the proposed study is approved, the Louisiana Tumor Registry will be assigned as the VTR Contact Registry. NCI will inform the investigator about the review outcome through the VTR Review Panel Outcome Letter sent via the VTR Request System.
- Initial Consultation about Request: The VTR Registry will meet with the investigator to advise them on study design and to answer questions. Meeting notes will be sent by the registry as guidance for the investigator's next step.
- Study Request Form (F2): The investigator completes and submits the Study Request Form (F2) in the VTR Request System.
- Case Counts, Budget, and Timeline Estimates: After receiving the Study Request Form (F2), the registry will establish the criteria to search their databases for eligible cancer cases. They will determine estimated case counts by demographic parameters, costs, and timeline for fulfillment of the request.
- Letter of VTR Services Available: The investigator will receive a letter from NCI VTR Program Staff with estimates of available cases, costs, and timeline for fulfillment. This letter may be included in funding applications.
- Project Documentation: When the investigator has obtained funding for the project, they will need to submit the required regulatory documentation for the proposed project, they will upload this documentation to the VTR Request System. The NCI VTR Coordinator will provide each investigator with the list of documentation needed, as it will vary by project.
- Fulfillment Stage: The registry acquires, processes, and sends de-identified, linked data and/or tissue to the investigator.
What Should Investigators Do after Obtaining Funding?
After the investigator receives the Letter of VTR Services Available and has obtained funding for their study, they will upload the regulatory documents to the VTR Request system. Investigators will be asked to submit all or a subset of the following documents depending on study design and planned tissue-based studies.
- Updated contact information
- Curriculum Vitae for Each Investigator
- Institutional Review Board (IRB): The SEER-Linked VTR Program requires investigators to submit their protocol to their IRB for determination of whether the study is exempt from review before receiving specimens and/or data. Because all tissue and data will be deidentified before the investigator receives them, and there will be no patient contact, the study is not considered human subjects research. NIH investigators will be required to submit an NIH Investigator Attestation Form.
- Copy of the Study Protocol
- Data Management and Sharing Plan: If the investigator's project is funded by the National Institutes of Health (NIH), the investigator must generate and submit a Data Management and Sharing Plan to comply with the NIH Data Management and Sharing Policy.
- Secured Funding: The investigator is responsible for securing funding and providing the NCI VTR Program Staff with documentation that funding was awarded for the project, the identity of the funder, and the start and end dates of the award.
- Institutional Certification: If the investigator requests tissue to be used for sequencing, then the investigator must have their institution sign an Institutional Certification and should comply with the NIH Data Management and Sharing Policy and the NIH Genomic Data Sharing Policy.
- Authentication and Authorization: The VTR Program is using the general SEER Data Request System for Research Plus data to authenticate and authorize each investigator who participates in the study. The email showing approval for SEER Research Plus Databases, serves as documentation.
- Material Transfer Agreement for the registry sending de-identified tissue.
If the details of the study and/or request have changed since case counts, budget, and timeline estimates were provided, investigators should correspond with NCI VTR Coordinator via the VTR Request System for guidance of the next steps. After study fulfillment has begun, no changes that impact study design may be made to the request. Any such changes to the request will need to be submitted as a new request.
For additional information, please see the FAQs.

